Abstract
Introduction
Notable advances have been made in multiple myeloma management (MM) in recent years. The monoclonal antibodies daratumumab (dara) and elotuzumab (elo) are integral in the management of relapsed/refractory MM and are moving into the frontline setting for transplant ineligible patients. Despite many trials evaluating the efficacy of these agents, there is no consensus on the optimal integration of these agents into the current paradigm of MM management. There is a paucity of data to guide the selection of one antibody over the other, and little is known about whether the use of one prior antibody alters the efficacy of other agents in subsequent lines of therapy. We retrospectively analyzed MM patients treated with both elo and dara during the course of their disease to assess whether the sequence of elo followed by dara or dara followed by elo led to better outcomes.
Methods
We reviewed the records of MM patients ≥ 18 years old who received both dara and elo at any time during treatment at MD Anderson Cancer Center. We evaluated the time to next treatment (TTNT), defined as the start date of one treatment to the start of the next treatment, and the overall response rate (ORR) (partial response or greater) to dara and elo. TTNT and ORR were calculated for dara when administered as the first antibody during treatment and to dara when administered as the second antibody (elo given during prior lines of treatment). We evaluated TTNT and ORR when elo was administered as the first antibody during treatment and to elo when administered as the second antibody (dara given during prior lines of treatment). The association with categorical patient variables (age, race, immunoglobulin class, number of prior lines of therapy, agent co-administered with the second monoclonal antibody given) was analyzed using Fisher exact tests. Outcomes were estimated using the Kaplan-Meier method and differences in survival among groups were assessed using two-sided log-rank tests.
Results
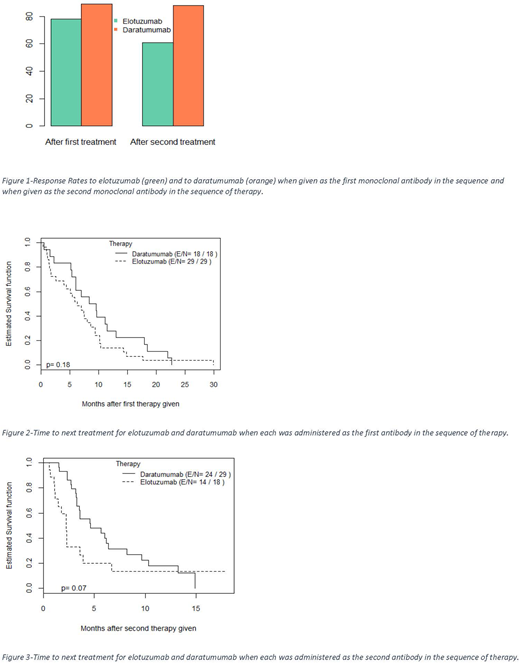
We identified 56 patients treated with both dara and elo. We excluded 6 for missing data; 56% of the 50 patients were male; 70% were Caucasian; 56% with IgG subclass paraprotein; 66% kappa light chain restricted. Patients were heavily pre-treated; most with >5 lines of therapy prior to initiation of dara or elo. Elo was the first agent received in 64% of patients. Independent of treatment order, the ORR to elo and dara were 72% and 88% respectively. When elo was administered prior to dara (elo first), the ORR was 78%, and 61% when administered after dara (elo second). When dara was administered prior to elo (dara first), the ORR was 89%, and 88% when administered after elo (dara second). There was no statistical difference in ORR (78% vs 89%) for the initial antibody given, but there was a significant difference in ORR (61% vs. 88%) to the agent given second (p=0.04). Of the variables assessed, only the antibody class had a statistically significant association with outcomes; IgG was associated with better responses when elo was administered as the second agent. TTNT analysis included 47 (of 50) evaluable patients. With elo given first, median TTNT was 6.28 months, and 2.23 months when given second. With dara given first, median TTNT was 8.97 months and 4.60 months when given second. There was no statistically significant difference in TTNT for either the first or second agent administered. There was a statistically significant improvement in TTNT in Caucasian patients compared to other races when elo was given first, but no other clinical factor association was statistically significant.
Conclusions
Our data, though retrospective and single-center, demonstrate that responses to dara are similar whether administered as the first or second monoclonal antibody used in the treatment of MM. However, prior treatment with dara negatively impacts responses to elo in subsequent lines of therapy. Similarly, independent of the sequence of administration, TTNT was prolonged with dara compared to elo. Our findings suggest that elo followed by dara may be the preferred sequence of monoclonal antibody therapy as responses to elo decreased when administered after dara, but responses to dara were stable irrespective of elo administration. These findings warrant further investigation particularly as dara is now being used more often in the frontline setting. Further evaluation is also needed to determine any association with additional patient factors such as cytogenetics.
Lee:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies Corporation: Consultancy; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Chugai Biopharmaceuticals: Consultancy; Takeda Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees. Patel:Poseida Therapeutics, Inc.: Research Funding; Takeda: Research Funding; Abbvie: Research Funding; Celgene: Research Funding. Thomas:Amgen Inc: Research Funding; Celgene: Research Funding; Bristol Myers Squibb Inc.: Research Funding; Array Pharma: Research Funding; Acerta Pharma: Research Funding. Orlowski:Janssen Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioTheryX, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Millenium Pharmaceuticals: Consultancy, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy; Poseida: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal